Ulcerative Colitis Treatment: What’s New and Next?



New and Next in UC Treatment
While many treatments for ulcerative colitis (UC) are available, they work better in some people than others. That’s why research is always ongoing to learn new ways of using existing drugs to treat the condition and to discover targets for new drugs.
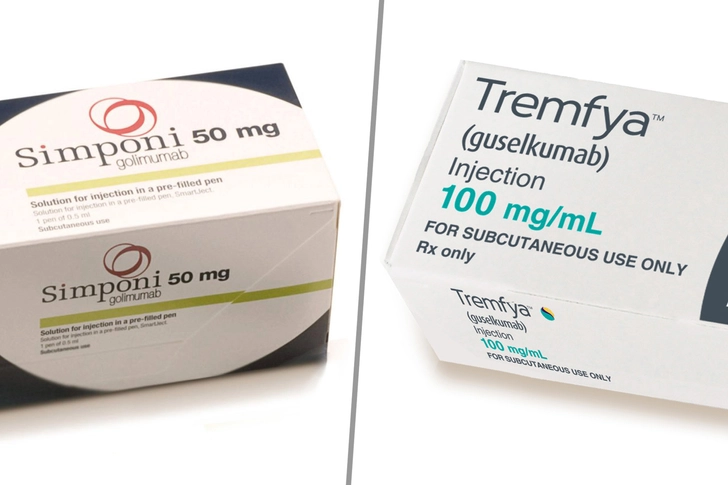
A Winning Combo
A 2023 study in Nature found that the immunosuppressant drugs golimumab (Simponi) and guselkumab (Tremfya) got better results when used together than separately. In the study, 83% of people saw reduced UC inflammation when they got both medicines. In those who took only golimumab, 61% responded. In the guselkumab-only group, the drug was effective for 75% of the people. These drugs have FDA approval to treat several types of arthritis.

Focus on Genes
Researchers are always on the lookout for genes that might play a role in UC. With this information, drug developers can explore medicines that might interfere with the gene in some way that would stop the disease. Recently, scientists have found at least two new candidate genes that could be good drug targets in the future.
Gut Bacteria: The Good and the Bad
Research is ongoing into the role that gut bacteria might play in UC. This could help doctors diagnose and treat the condition in the future. When scientists understand which bacteria help and hurt in UC, it’s possible they could develop treatments that boost the good bacteria or reduce the bad.
A Closer Look at Cytokines
Some UC drugs such as adalimumab (Humira) target cytokines, substances that have a critical role in UC inflammation. But not everyone benefits from these drugs. New research could explain why. Cytokine levels in UC tend to differ from person to person, depending on both the individual and the stage of their disease. This discovery suggests that doctors might need to figure a person’s distinct “cytokine profile” into treatment choices.
New Drug Target: IL-22
One cytokine that seems to be a major player in UC is interleukin 22, or IL-22. When it comes to UC, this pesky cytokine gets around. It interferes with chemical reactions in the body, gene activity, and gut bacteria – all of which affect UC. Drugs that block interleukins are already available to treat some autoimmune conditions, but none of them target 22. Some researchers are now focused on finding a drug that shuts down this cytokine.

New Drug Target: uPA
New research finds that levels of a protein called uPA (urokinase-type plasminogen activator) are higher in people with UC and highest in those who have the most severe disease. When scientists gave a uPA-blocking drug to mice with UC, their condition improved a lot. These findings could help pave the way for a UC treatment that blocks this protein in people.
Poop Transplant
Studies continue into the use of other people’s poop to treat UC, a procedure called fecal transplant. In some people, this puts UC into remission. In theory, feces that come from someone who does not have UC may contain a better balance of gut bacteria. In the gut of someone with UC, the foreign feces could change the bacterial makeup for the better. Researchers are exploring whether the treatment is best delivered through a pill or an enema.

Chinese Medicine
Western doctors typically turn to prescription drugs to treat UC. But new research provides early support for an ancient Eastern approach. Scientists gave xianglian pills to mice with UC. This herbal supplement lengthened the colon and reduced disease activity, disease-related changes in the colon, and levels of several inflammatory proteins. More research is needed to see if it would work in humans.
New Drug Coming Down the Pike
Clinical trials are looking good for a new drug called etrasimod. In UC, immune cells travel to the lining of your colon, where they cause inflammation and damage. Etrasimod, a once-daily pill, regulates the movement of certain immune cells in your bloodstream, which keeps them from collecting in the lining of your colon. Up to a third of the people who took it went into remission that lasted at least a year.
IMAGES PROVIDED BY:
- DigitalVision / Getty Images
- Janssen Immunology
- Westend61 / Getty Images
- iStock / Getty Images Plus / Getty Images
- Juan Gaertner / Science Source
- Scott Camazine / Medical Images
- E+ / Getty Images
- Carol Werner / Medical Images
- iStock / Getty Images Plus / Getty Images
- Science Photo Library / Getty Images
SOURCES:
Nature Reviews Gastroenterology & Hepatology: “Two therapeutic antibodies better than one.”
European Journal of Human Genetics: “A family with ulcerative colitis maps to 7p21.1 and comprises a region with regulatory activity for the aryl hydrocarbon receptor gene.”
Cellular and Molecular Gastroenterology and Hepatology: “PTPN2 Is a Critical Regulator of Ileal Paneth Cell Viability and Function in Mice.”
Scientific Reports: “Results and lessons learned from the sbv IMPROVER metagenomics diagnostics for inflammatory bowel disease challenge.”
Autoimmunity Reviews: “The influence of cytokines on the complex pathology of ulcerative colitis,” “Urokinase-type plasminogen activator blockade ameliorates experimental colitis in mice.”
Biomedicine and Pharmacotherapy: “Role of Interleukin-22 in ulcerative colitis.”
The Lancet: “Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial,” “Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): two randomised, double-blind, placebo-controlled, phase 3 studies.”
Journal of Ethnopharmacology: “Xianglian Pill attenuates ulcerative colitis through TLR4/MyD88/NF-κB signaling pathway.”
Immunotherapy: “Etrasimod for the treatment of ulcerative colitis.”