- Overview
- Symptoms
- Causes & Risks
- Screening & Testing
- Diagnosis
- Treatment
- How HIV Affects the Body
- Opportunistic Infections
- Complications
- Living With
- Dating & Relationships
- Support & Resources
- Prevention
- Appointment Prep
- View Full Guide
Advances in HIV Treatment


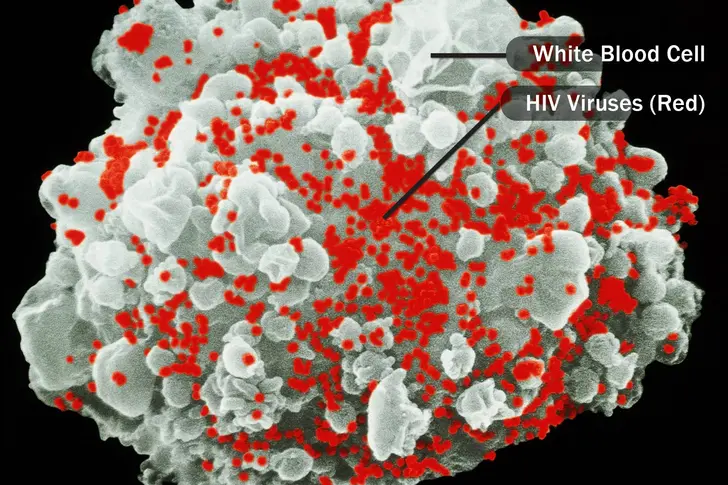
Human Immunodeficiency Virus (HIV)
HIV spreads through unprotected sex, contaminated needles, and from mother to child. It attacks your immune cells, which protect your body from germs. In six steps, HIV can:
- Enter immune cells called T lymphocytes.
- Change viral RNA with a reverse transcriptase protein into DNA.
- Insert the viral DNA into your DNA.
- Make many copies of itself.
- Assemble new viruses.
- Break out of your cell to enter new ones.
Approach to HIV Treatment
HIV therapies have come a long way, with more than 50 types of antiretroviral therapies (ART) that help lower your risk of spreading the virus. It's not a cure, but ART can help you live a longer, healthier life with HIV. ART often combines more than one medicine into one or more pills. But ART can stop working, or some people may get tired of taking pills and opt for an injection.

Long-Acting ART
Instead of taking pills every day, you can try long-acting ART injections. But first, you take pills to reach an "undetectable" amount of virus. Then, you'll take a shot every one or two months which combines cabotegravir and rilpivirine (Cabenuva).
Ibalizumab (Trogarzo)
If other HIV treatments aren't working, ibalizumab-uiyk can treat multidrug-resistant HIV and block HIV from entering your T cells. You'll get this infusion through an IV in your doctor's office or clinic to raise the medicine concentration in your body. Then, you'll go back for infusions every two weeks.
Lenacapavir (Sunlenca)
Lenacapavir is another small molecule that can treat multidrug-resistant HIV, if other HIV treatments don't work for you. This medication can block HIV from making new copies of itself and lower your risk of spreading HIV. You'll start with pills to get the correct medicine dose into your body first. Then, you'll take a shot twice yearly.

HIV Vaccines
Early clinical trials show promise for some types of vaccines against HIV. The vaccines use bits of harmless, lab-made protein or RNA that mimic HIV. Even though the pieces of protein or RNA can't infect you, they train your immune system to fight off HIV before you get infected. A few of the trials show some vaccines can help your body make antibodies to block HIV from infecting your T cells.

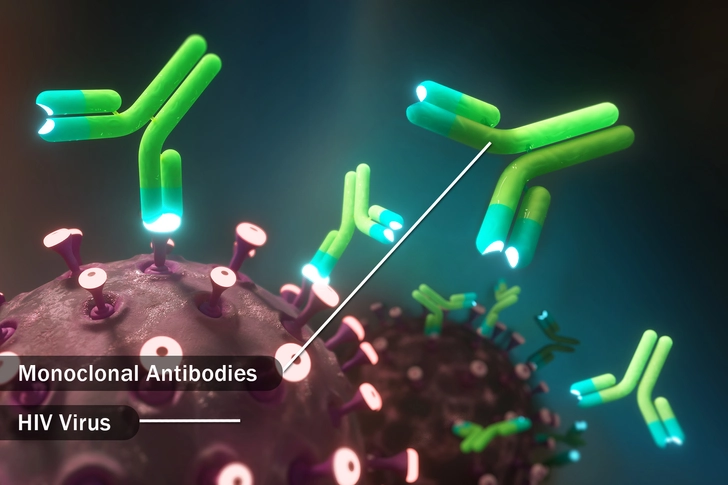
bNAbs (Broadly Neutralizing Antibodies)
Broadly neutralizing antibodies (bNAbs) are lab-made antibodies that can block many types of HIV from entering your T cells. Instead of your body making them, you'll get the antibodies through a shot or IV.
It’s possible that you’ll only need to do this once every six to 12 months. Researchers are still studying whether they can treat HIV this way.

Gene Editing
To make copies of itself, HIV inserts its own viral DNA to hijack certain human genes. Someday, doctors might use gene technology to turn off or edit out some of these genes.
A recent study identified more than 60 human genes that could potentially be used in this treatment. Another study used a gene editing method called CRISPR to cut out the virus from cells for a "cure." But more research is needed to know if this method is safe.
Doravirine Plus Islatravir
If other HIV medications haven't worked, a new combination of doravirine plus islatravir may help. This combination pill is being studied in clinical trials. The combined HIV medicines work by stopping HIV from making copies of itself.
Recent trials show you may only need to take one tablet a day.
Where Can I Find More Information About ART?
With so many HIV treatments to choose from, you may want to do more research. You can always look up medications at WebMD. Or you can check out a few U.S. government resources at:
IMAGES PROVIDED BY:
- NIBSC / Science Source
- Francis Sheehan / Science Source
- BrianAJackson / Getty Images
- Tetra Images / Getty Images
- SCI-COMM STUDIOS / Science Source
- Bing Guan / Bloomberg Creative / Getty Images
- Alernon77 / Getty Images
- MARK GARLICK / SCIENCE PHOTO LIBRARY / Getty Images
- Jure Gasparic / EyeEm / Getty Images
- Will & Deni McIntyre / Science Source
SOURCES:
Journal of Antimicrobial Chemotherapy: “Long-acting antiretrovirals: a new era for the management and prevention of HIV infection.”
Drugs: “Advances in long-acting agents for the treatment of HIV infection.”
Antimicrobial Agents and Chemotherapy: “Ibalizumab, a novel monoclonal antibody for the management of multidrug-resistant HIV-1 infection.”
HIV.gov: “FDA-Approved HIV Medicines,” “HIV and AIDS: The Basics,” “The HIV Life Cycle,”“HIV Treatment: The Basics,”“Islatravir,” “Lenacapavir.”
Current Opinion in HIV and AIDS: “Lenacapavir: a first-in-class HIV-1 capsid inhibitor.”
Nature Communications: “A functional map of HIV-host interactions in primary human T cells.”
International AIDS Vaccine Initiative: “HIV antibodies.”
UCSF Health: “HIV/AIDS program.”
FDA: “FDA Approves Cabenuva and Vocabria for the Treatment of HIV-1 Infection,” “FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention,” "HIV and AIDS: Medicines to Help You."
Viruses: "New Therapies and Strategies to Curb HIV Infections with a Focus on Macrophages and Reservoirs."
UpToDate: "Overview of antiretroviral agents used to treat HIV."
Emerging Microbes and Infections: "Broadly neutralizing antibodies for HIV-1: efficacies, challenges and opportunities."
Lancet: "Switch to fixed-dose doravirine (100 mg) with islatravir (0·75 mg) once daily in virologically suppressed adults with HIV-1 on antiretroviral therapy: 48-week results of a phase 3, randomised, open-label, non-inferiority trial."